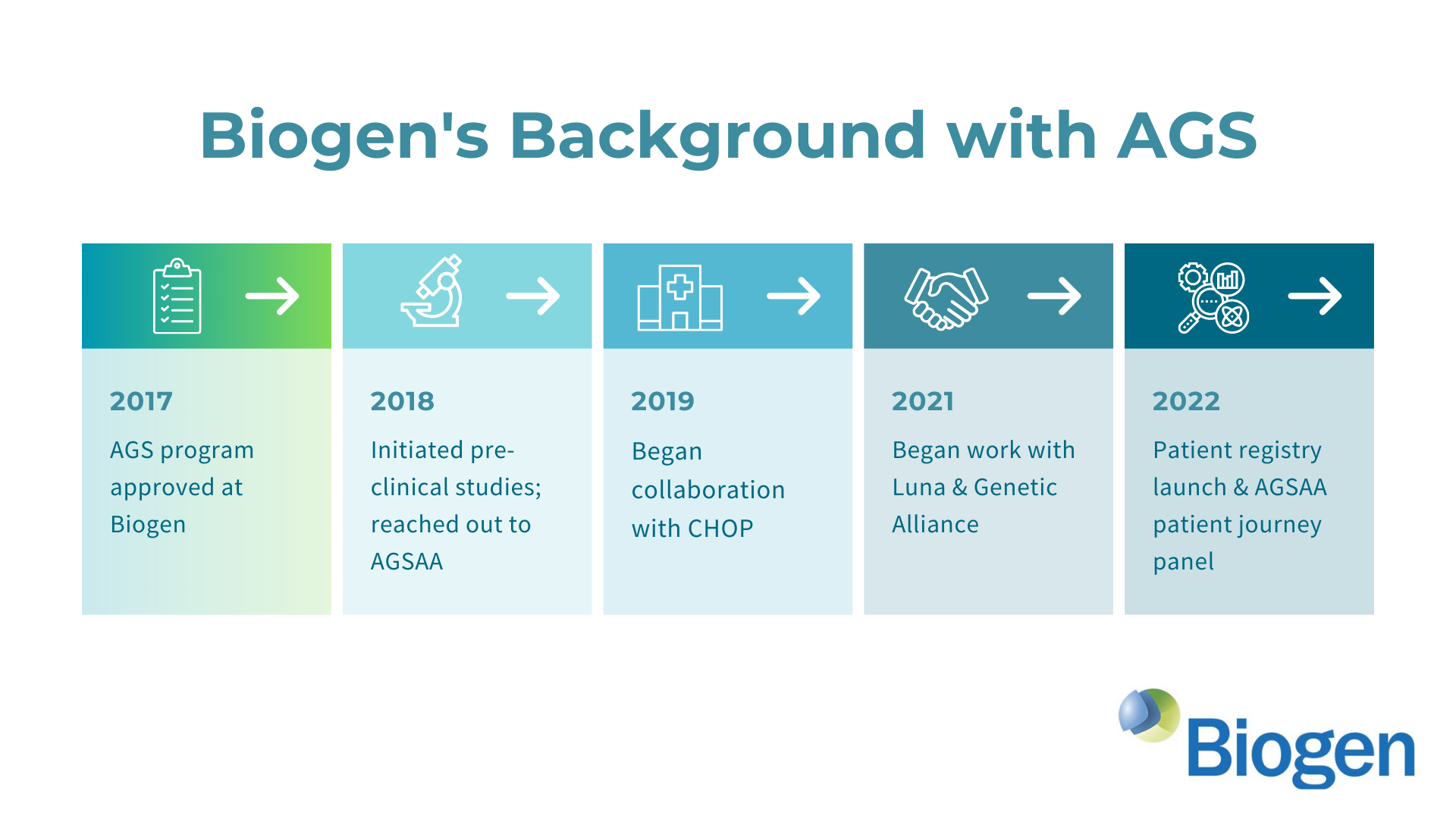
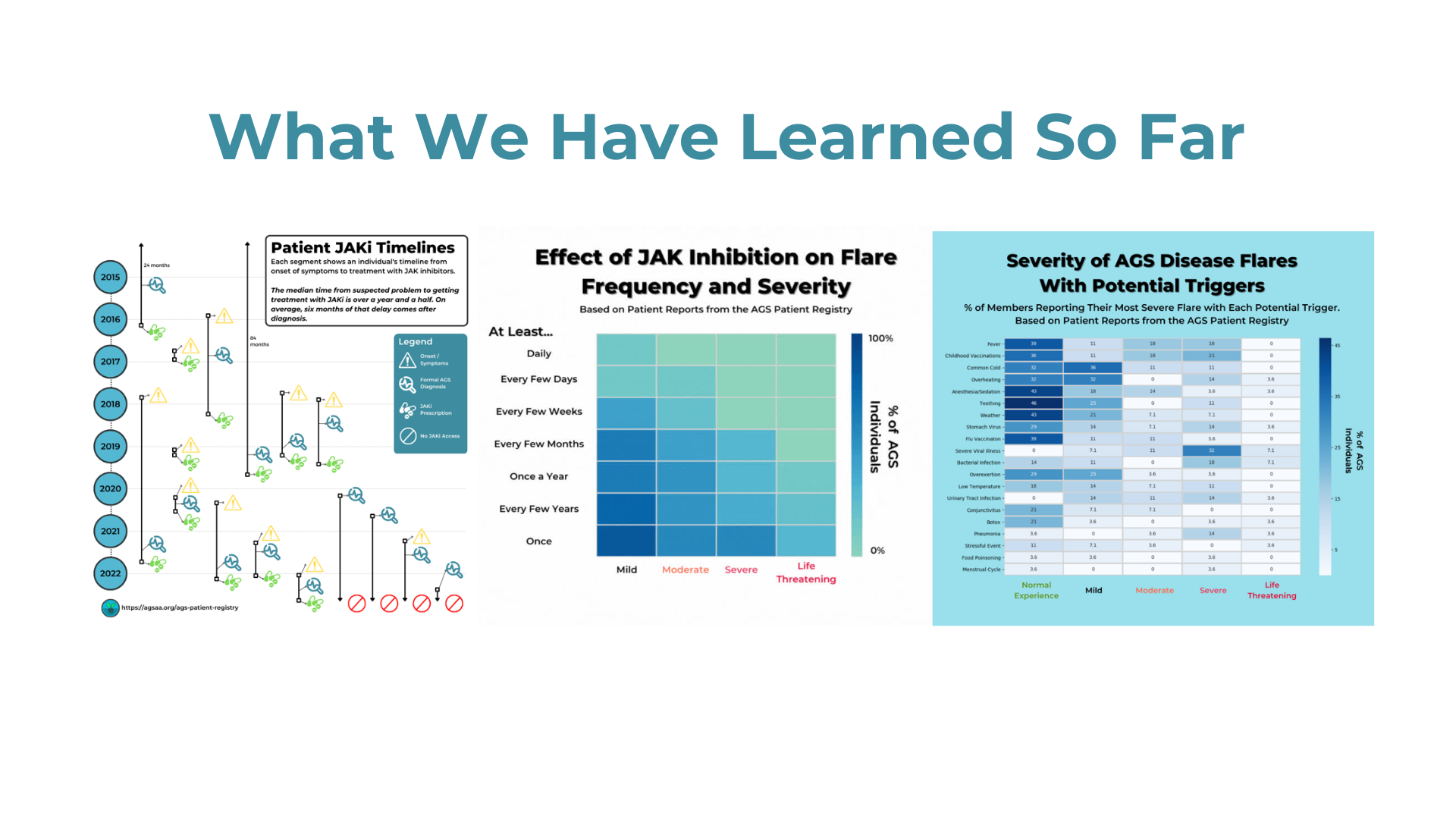
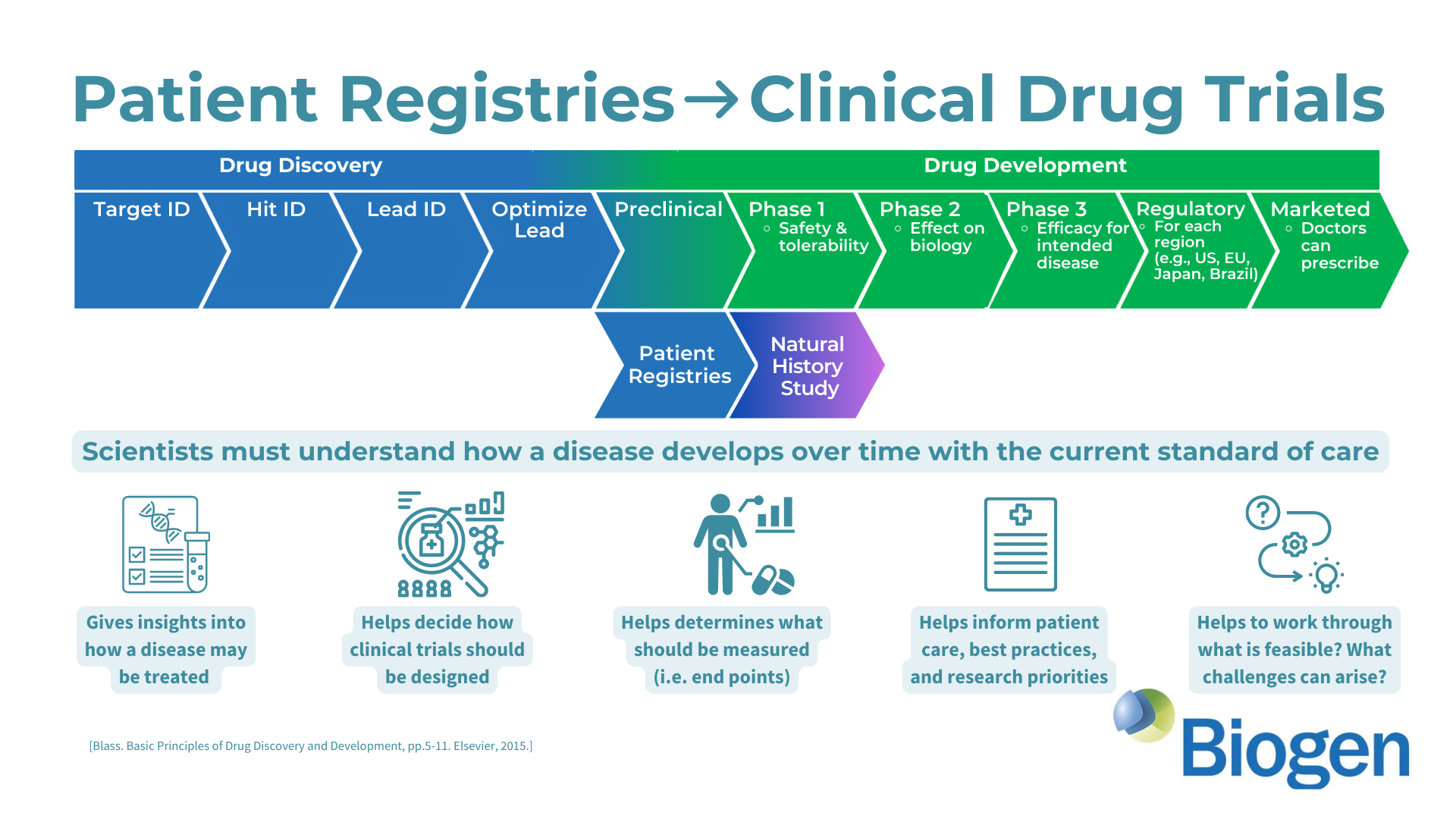
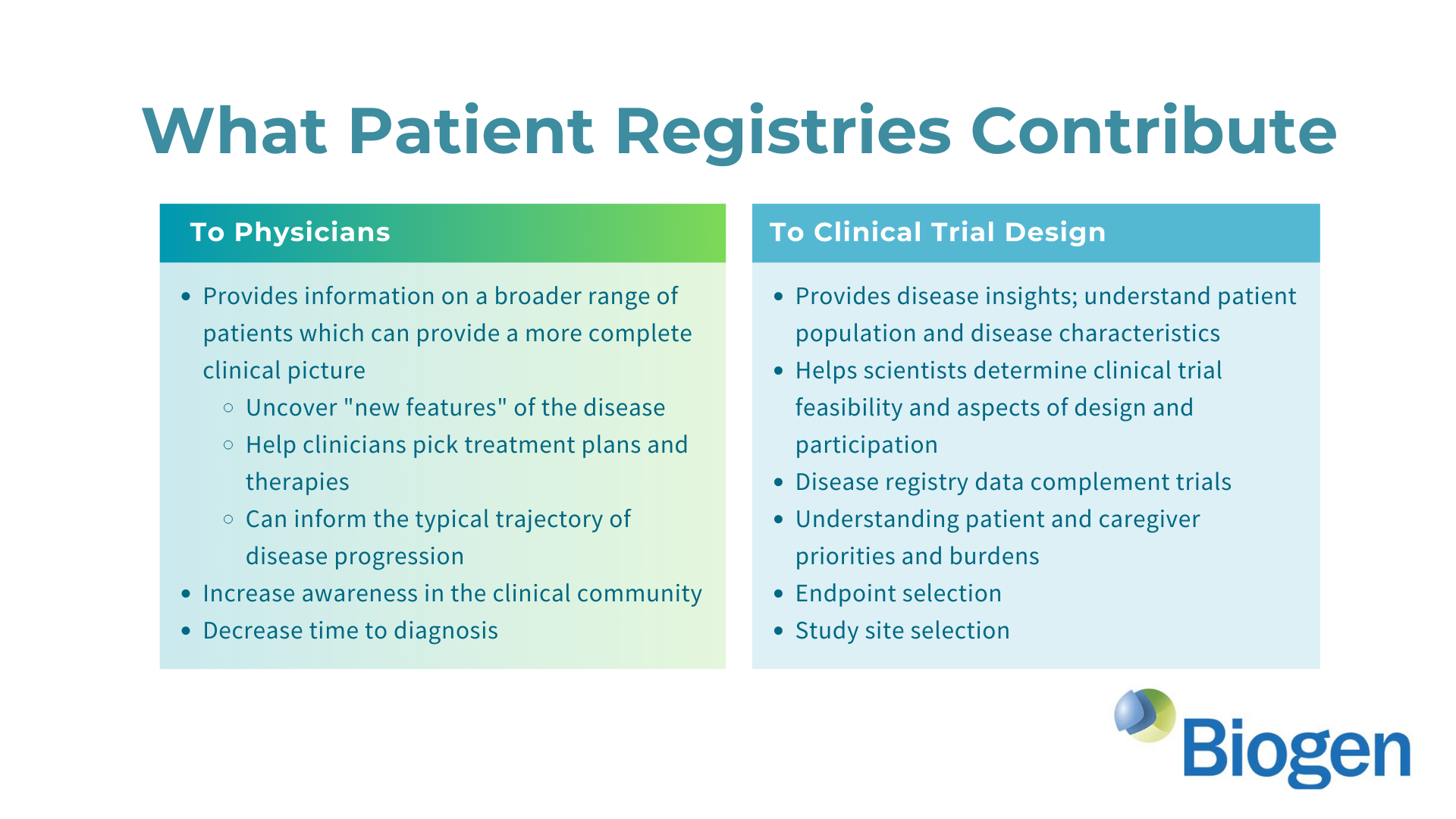
The AGS Advocacy Association (AGSAA) has been working with the pharmaceutical company Biogen (biogen.com) to ensure the development an effective clinical trial for Aicardi-Goutieres Syndrome (AGS). We’re excited to share some details about this collaboration in our first webinar, and we have an earnest request for all AGS families. Please watch our webinar in which we explain the state of Biogen’s program for AGS and how a new AGS patient registry study and survey can help Biogen develop a forthcoming clinical trial.
A study managed by the pharmaceutical company Biogen to inform and improve the development of a clinical trial for Aicardi-Goutieres Syndrome. Your survey responses in this study will be anonymized and shared with Biogen.
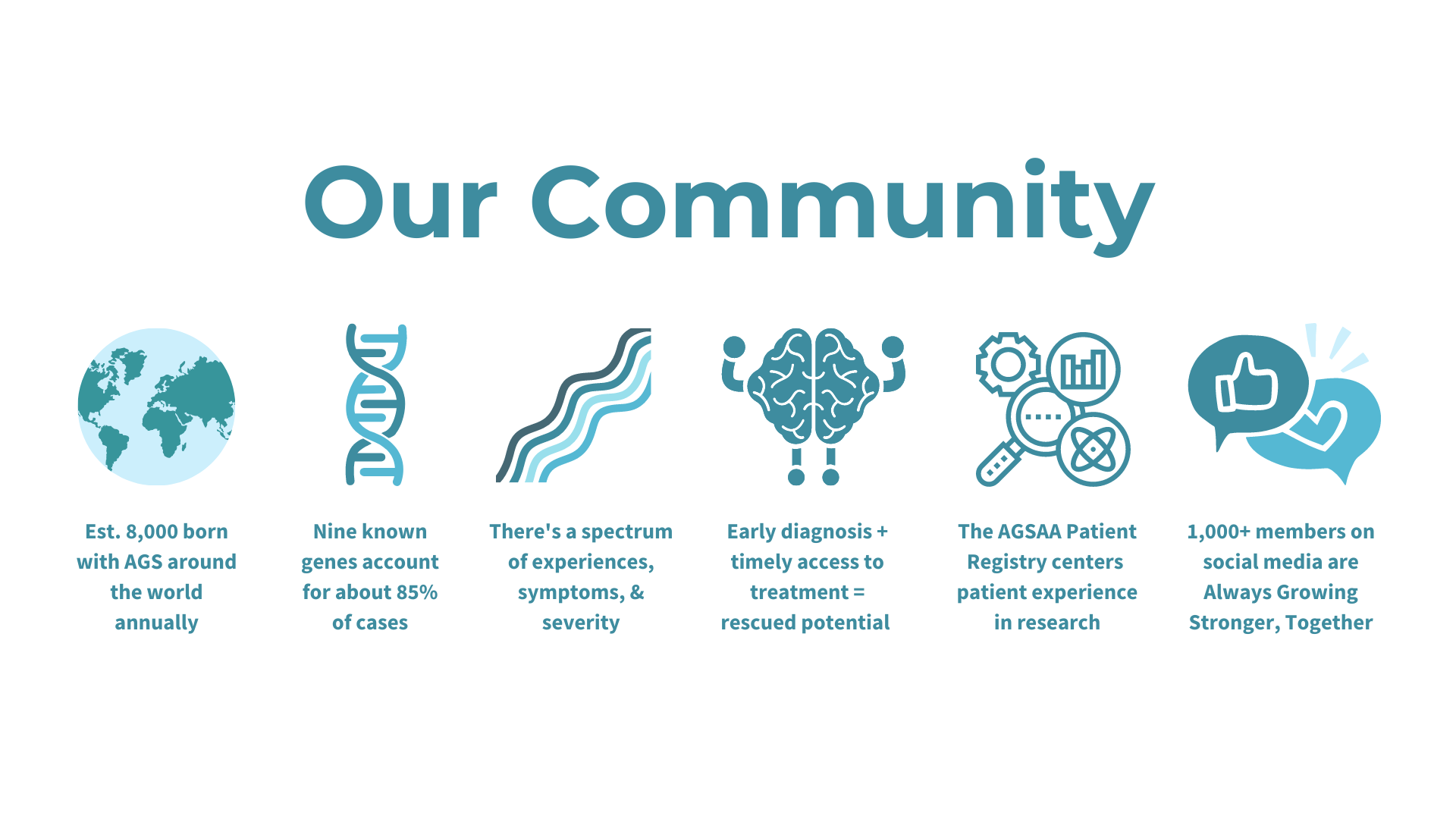
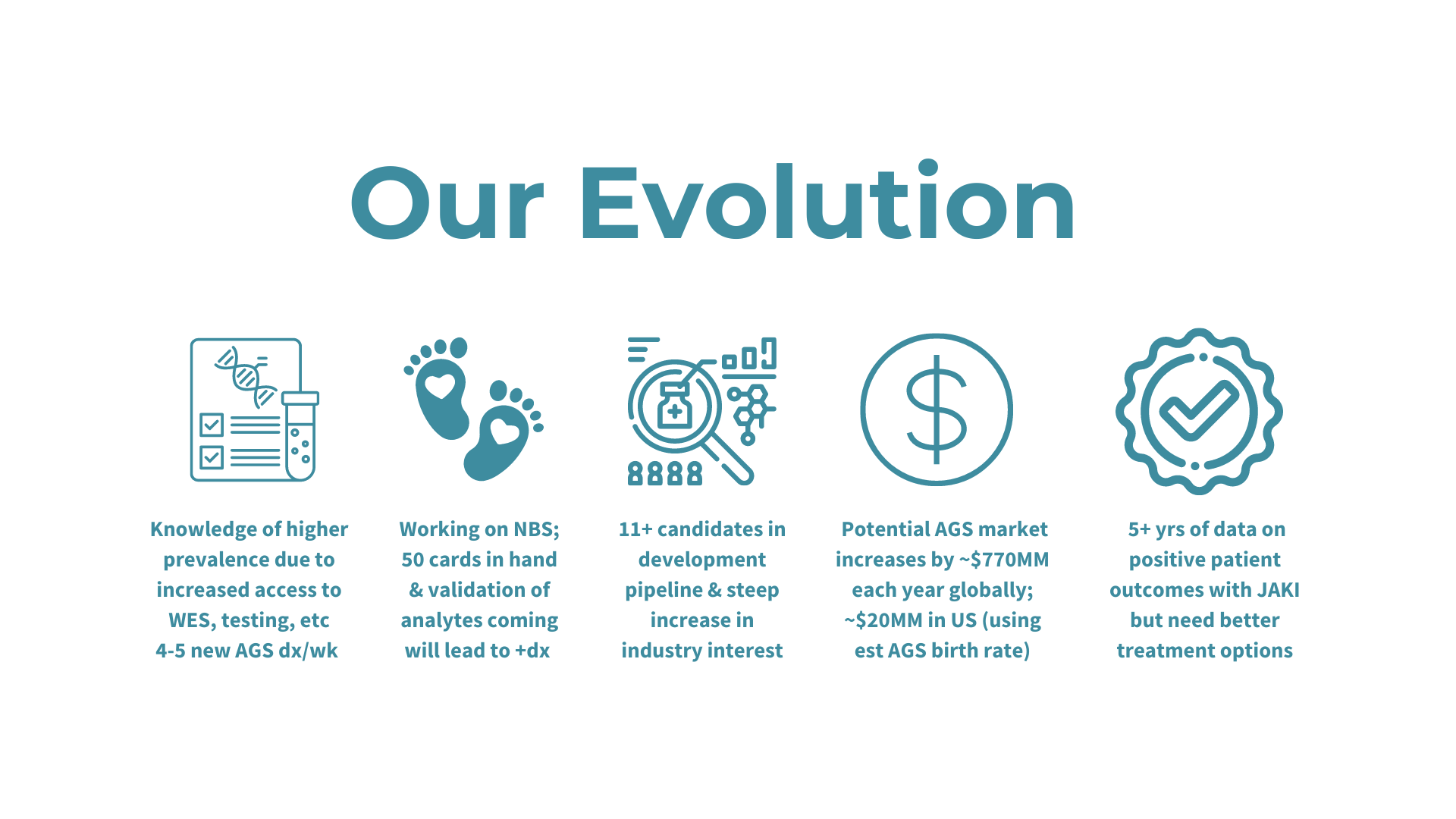
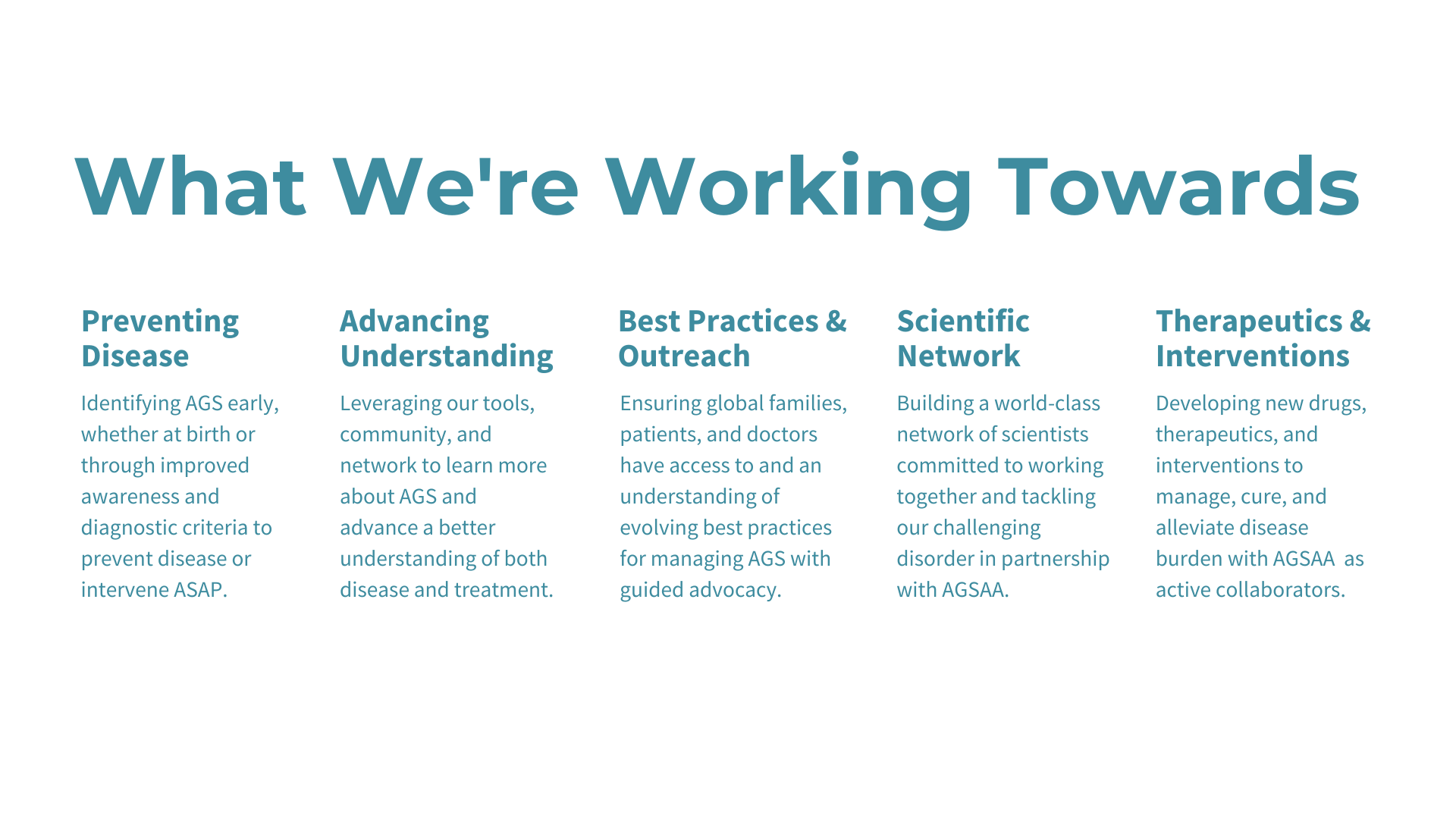
Presentation Slides